Fda Approved Covid Vaccines Philippines
They submitted today and. The Food and Drug Administration FDA recently approved the emergency use of Russias Sputnik Light COVID-19 vaccine in the Philippines.

Sinopharm Seeks Eua For Covid 19 Vaccines In Philippines
1 day agoMANILA Philippines The Phiippines Food and Drug Administration FDA has granted an emergency use authorization EUA to Russias single-dose COVID-19 vaccine.

Fda approved covid vaccines philippines. 1 day agoIn March the Philippines FDA granted the emergency use authorization to use Russias two-dose Sputnik V coronavirus vaccine. Acting FDA Commissioner Janet Woodcock MD. Pfizer and BioNTech which developed one of the three COVID-19 vaccines available in the US in May completed their application for full FDA approval for use in.
Russias single-dose COVID-19 vaccine was the second Russia-made COVID-19 vaccine to be granted emergency use authorization EUA in the country next to Sputnik V COVID-19 vaccine. 1 day agoThe Philippines also has approved several Covid-19 vaccines including Sinovac Sinopharm AstraZeneca and Pfizer among others. Novavax Inc needs to submit several more documents for its 2-dose COVID-19 vaccines application for emergency use authorization in the Philippines the Food and Drug Administration said on Monday.
A COVID-19 vaccine must be proven to be at least 60 to 70 effective before the Philippines Food and Drug Administration FDA approves its use in the country FDA Director-General Eric Domingo said. Generally it should be 60 to 70 effective. 6 hours agoPhilippine authorities announced that the Food and Drug Administration FDA has approved the emergency use of Russias Sputnik Light COVID-19 vaccine.
FDA Philippines Grants Emergency Use Authorization to Pfizer-BioNTech COVID-19 Vaccine Today the Food and Drug Administration FDA grants its first Emergency Use Authorization EUA to Pfizer-BioNTech COVID19 Vaccine BNT162b2 Suspension for IM. Pfizer-BioNTech COVID-19 Vaccine BNT162b2 ChAdOx1-Srecombinant COVID-19 Vaccine AstraZeneca SARS-CoV-2 Vaccine Vero Cell Inactivated Coronavac Sputnik V Gam-COVID-Vac COVID-19 Vaccine. That is the minimum requirement Domingo said in an ANC interview.
And the Director of FDAs Center for Biologics Evaluation and Research Peter Marks MD PhD discuss the. MANILA Philippines Local regulators on Monday said the American drugmaker Moderna has applied for emergency use approval in the Philippines for its COVID-19 vaccine. The Philippines has administered over 30 million doses of COVID-19 vaccines.
The FDA amended the EUAs for the Pfizer-BioNTech and Moderna COVID-19 Vaccines to allow an additional dose in certain immunocompromised individuals. 56 minutes agoMANILA Philippines Pfizer-BioNtech will have to apply for a Certificate for Product Registration CPR for its COVID-19 vaccines to be given full approval to be used in the Philippines. The Pfizer-BioNTech jab has been authorized for emergency use since December with more than 204 million people in the US receiving.
Over 13 million people have been fully vaccinated against COVID-19 so far. The United States Food and Drug Administration has given full approval on Monday to the Pfizer-BioNTech COVID-19 vaccine for use in people over age of 16 a first such approval of a COVID-19 jab. The government aims to vaccinate up to 70 million people this year.
15 hours agoPfizer needs to apply first before its COVID-19 vaccine could be given full use approval in the Philippines according to the head of the Food and Drug Administration FDA on Tuesday. The American biotech firm last week applied for. Janssen COVID-19 Vaccine Ad26COV2-S recombinant Whole Virion Inactivated Corona Virus Vaccine Covaxin.
List of COVID-19 Vaccines Authorized by the FDA. The Philippines also has approved several COVID-19 vaccines including Sinovac Sinopharm AstraZeneca and Pfizer among others. The COVID-19 vaccine developed by American drug firm Moderna is the seventh vaccine to be granted emergency use approval in the Philippines.
The Philippines has administered over 30 million doses of Covid-19 vaccines. 18 hours agoEven though the FDA has approved the PfizerBioNtech Covid-19 vaccine for people age 16 and older the agency emphasizes that this does not mean the vaccine can be used off-label in.

Psmid Philippines Psmidorg Twitter
Johnson Johnson Applies For Emergency Covid 19 Vaccine Use In The Philippines Reuters
Department Of Health Philippines It Pays To Know The Right Information It Pays To Know The Right Information Read And Know The Right Information On The Covid 19 Vaccines Vaccines Protect

Fda Philippines Grants Emergency Use Authorization To Pfizer Biontech Covid 19 Vaccine Food And Drug Administration
Fda Warning About J J Vaccine Creates More Challenges For Fighting Covid Crisis
Novavax Applies For Emergency Use Of Its Covid 19 Vaccine In Philippines
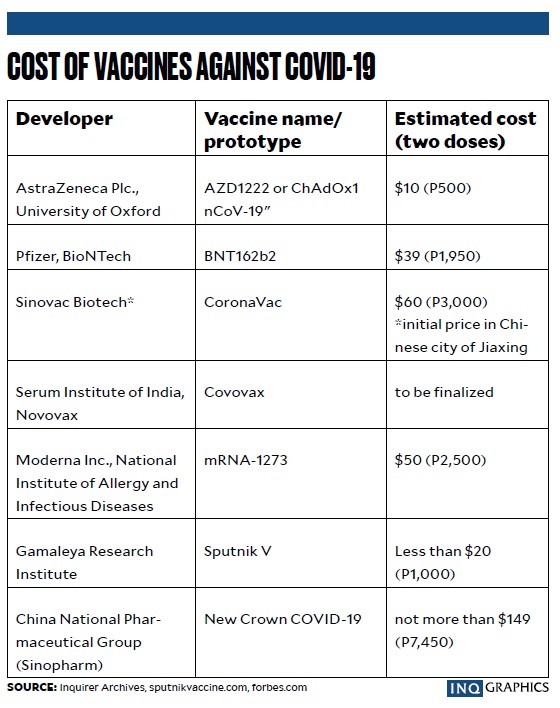
Varying Prices Of Sinovac Covid 19 Vaccine Raise Alarm Inquirer News

Know Your Vaccines Vaccine Matrix Current Evidence Department Of Health Website

Timeline The Philippines 2021 Covid 19 Vaccine Plan

Russia S Sputnik V Vaccine Approved For Emergency Use In The Philippines

U S Welcomes Arrival To The Philippines Of U S Supported Covax Vaccines U S Embassy In The Philippines
Https Www Fda Gov Ph Wp Content Uploads 2021 03 Eua Sinovac Website 3 1 Pdf

Know Your Vaccines Vaccine Matrix Current Evidence Department Of Health Website

Fda Advisory No 2021 0664 Public Health Warning On Fake Covid 19 Vaccines Food And Drug Administration

Faqs Emergency Use Authorization Department Of Health Website

Fda Covid 19 Updates Food And Drug Administration
Philippines Clears Sinovac Shots But Not For Health Workers Bloomberg
Department Of Health Philippines Vaccine Tracker Covid 19 Vaccines Selection And Approval As Of 19 March 2021 Information You Need To Know About The Selection Process And Approval Of The Covid 19 Vaccines

Fda Approves 5 Rapid Test Kits For Covid 19 Philippine News Agency


Post a Comment for "Fda Approved Covid Vaccines Philippines"